TASIGNA®
Important note: Before prescribing, consult full prescribing information.
Presentation: Capsules containing 150 mg nilotinib (as hydrochloride monohydrate): White to yellowish powder in red opaque hard gelatin capsules, size 1, with black axial imprint “NVR/BCR”.
Capsules containing 200 mg nilotinib (as hydrochloride monohydrate):
White to yellowish powder in light yellow opaque hard gelatin capsules, size 0, with red axial imprint “NVR/TKI”.
Indications:
• First-line treatment of adult patients and paediatric patients aged 2 years and over with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase.
• Treatment of Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic or accelerated phase in adult patients resistant to or experiencing high toxicity on prior treatment
with imatinib.
• Treatment of Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase in paediatric patients aged 2 years and over resistant to or intolerant to prior treatment with
imatinib.
Dosage:
Therapy should be initiated by a physician experienced in the treatment of patients with CML. Tasigna should be taken twice daily at approximately 12-hour intervals. No food should be consumed for at
least 2 hours before and at least 1 hour after a dose is taken.
The capsules should be swallowed whole with water and should not be chewed or sucked. They should not be opened.
For patients unable to swallow capsules the contents of each capsule may be dispersed in one teaspoon of apple sauce (puréed apple) and should then be taken immediately. No more than one teaspoon of
apple sauce and no food other than apple sauce must be used.
Hands should be washed after contact with the capsules. Ensure that the powder contained in the capsules (e.g. if a capsule is damaged) is not inhaled and does not come into contact with the skin or mucous membranes.
In case of contact with the skin the area should be washed with soap and water. In case of contact with the eyes they should be rinsed with water. If any of the capsule powder is spilled,
it should be wiped up using gloves and a moist disposable towel and properly disposed of in a sealed container.
First-line treatment of Ph+ CML-CP in adult patients
The recommended dose of Tasigna is 300 mg twice daily. Treatment should be continued for as long as clinical benefits are observed or until unacceptable toxicity occurs.
Resistance or intolerance to prior treatment with imatinib (Ph+ CML-CP and CML-AP) in adult patients
The recommended dose of Tasigna is 400 mg twice daily. Treatment should be continued for as long as clinical benefits are observed or until unacceptable toxicity occurs.
Dosage in paediatric patients with newly diagnosed Ph+ CML-CP or resistant or intolerant Ph+ CML-CP
The dosage in paediatric patients is individualised and based on body surface area (mg/m2). The recommended Tasigna dose is 230 mg/m2 twice daily, rounded to the nearest 50 mg dose (up to a maximum dose of 400 mg) (see Table 1). Different strengths of Tasigna hard capsules can be combined
to attain the desired dose. Treatment should be continued for as long as clinical benefits are observed or until unacceptable toxicity occurs.
There is no experience with treatment of paediatric patients under 2 years of age.
Table 1 Dosage regimen for Tasigna 230 mg/m2 twice daily
| Body surface area |
Dose in mg
(twice daily) |
| Up to 0.32 m2 |
50 mg |
| 0.33-0.54 m2 |
100 mg |
| 0.55-0.76 m2 |
150 mg |
| 0.77-0.97 m2 |
200 mg |
| 0.98-1.19 m2 |
250 mg |
| 1.20-1.41 m2 |
300 mg |
| 1.42-1.63 m2 |
350 mg |
| ≥1.64 m2 |
400 mg |
Dosing in pediatric patients is individualized and is based on body surface area (mg/m2), rounded to the nearest 50 mg. Kindly note that the available dosage forms and strengths are 150 mg and 200 mg
capsules, 50 mg capsules is not registered in this country”
Monitoring recommendations
A baseline ECG is recommended prior to initiating therapy with Tasigna. The ECG should be repeated after 7 days and as clinically indicated. Hypokalaemia and hypomagnesaemia must be corrected
prior to administration of Tasigna. The potassium and magnesium levels in the blood should be regularly monitored during treatment, especially in patients with an increased risk of abnormalities of
these electrolytes .
Dose adjustment due to toxicity
Adult patients in chronic phase: In the event of haematological toxicity (neutrophil count <1x109/l and/or platelet count <50x109/l) Tasigna should be temporarily withheld and treatment should be
resumed at the original dose within 2 weeks of recovery of haemopoiesis. If levels remain low, continued treatment at a reduced dose of 400 mg once daily should be considered.
Adult patients in accelerated phase: In the event of haematological toxicity (neutrophil count <0.5x109/l and/or platelet count <10x109/l) Tasigna should be temporarily withheld and treatment should be
resumed at the original dose within 2 weeks of recovery of haemopoiesis (neutrophil count >1.0x109/l and/or platelet count >20x109/l). If levels remain low, continued treatment at a reduced dose of 400
mg once daily should be considered.
Paediatric patients in chronic phase: In the event of haematological toxicity (neutrophil count
<1x109/l and/or platelet count <50x109/l) Tasigna should be temporarily withheld and treatment should be resumed at the original dose within 2 weeks of recovery of haemopoiesis (neutrophil count
1.5x109/l and/or platelet count >75x109/l). If levels remain low, continued treatment at a reduced dose of 230 mg/m2 once daily should be considered. If an event occurs following dose reduction, treatment discontinuation should be considered.
If clinically significant moderate or severe non-haematological toxicity develops, treatment should be interrupted and the patient monitored and treated accordingly. If the prior dose was 300 mg twice
daily in adult patients newly diagnosed with CML-CP or 400 mg twice daily in adult patients with resistant or intolerant CML-CP and CML-AP or 230 mg/m2 twice daily in paediatric patients, treatment may be resumed at 400 mg once daily in adult patients or at 230 mg/m2 once daily in paediatric patients once the toxicity has resolved. If the prior dose was 400 mg once daily in adult patients or
230 mg/m2 once daily in paediatric patients, treatment should be discontinued. If clinically indicated, re-escalation of the dose in adult patients to 300 mg (newly diagnosed Ph+ CML-CP) or 400 mg
(resistant or intolerant)
Ph+ CML-CP and CML-AP) twice daily or to 230 mg/m2 twice daily in paediatric patients should be attempted.
Elevated serum lipase: In adult patients with elevated lipase (grade 3 or 4) treatment should be reduced to 400 mg once daily or interrupted. In paediatric patients treatment must be interrupted until a
return to grade 1. Thereafter, treatment can be resumed at 230 mg/m2 once daily if the prior dose was
230 mg/m2 twice daily. If the prior dose was 230 mg/m2 once daily, treatment should be discontinued. Serum lipase levels must be monitored monthly or as clinically indicated.
Elevated bilirubin and hepatic transaminases: In adult patients with elevated bilirubin or hepatic transaminases (grade 3 or 4) treatment should be reduced to 400 mg once daily or interrupted. For grade
2 bilirubin elevations or grade 3 hepatic transaminase elevations in paediatric patients treatment must be interrupted until a return to grade 1. Thereafter, treatment can be resumed at
230 mg/m2 once daily if the prior dose was 230 mg/m2 twice daily. If the prior dose was 230 mg/m2 once daily and the return to grade 1 takes longer than 28 days, treatment should be discontinued.
Bilirubin and hepatic transaminase levels should be monitored monthly or as clinically indicated. Dosage in Ph+ CML-CP patients who have achieved a sustained deep molecular response (MR 4.5) on
Tasigna
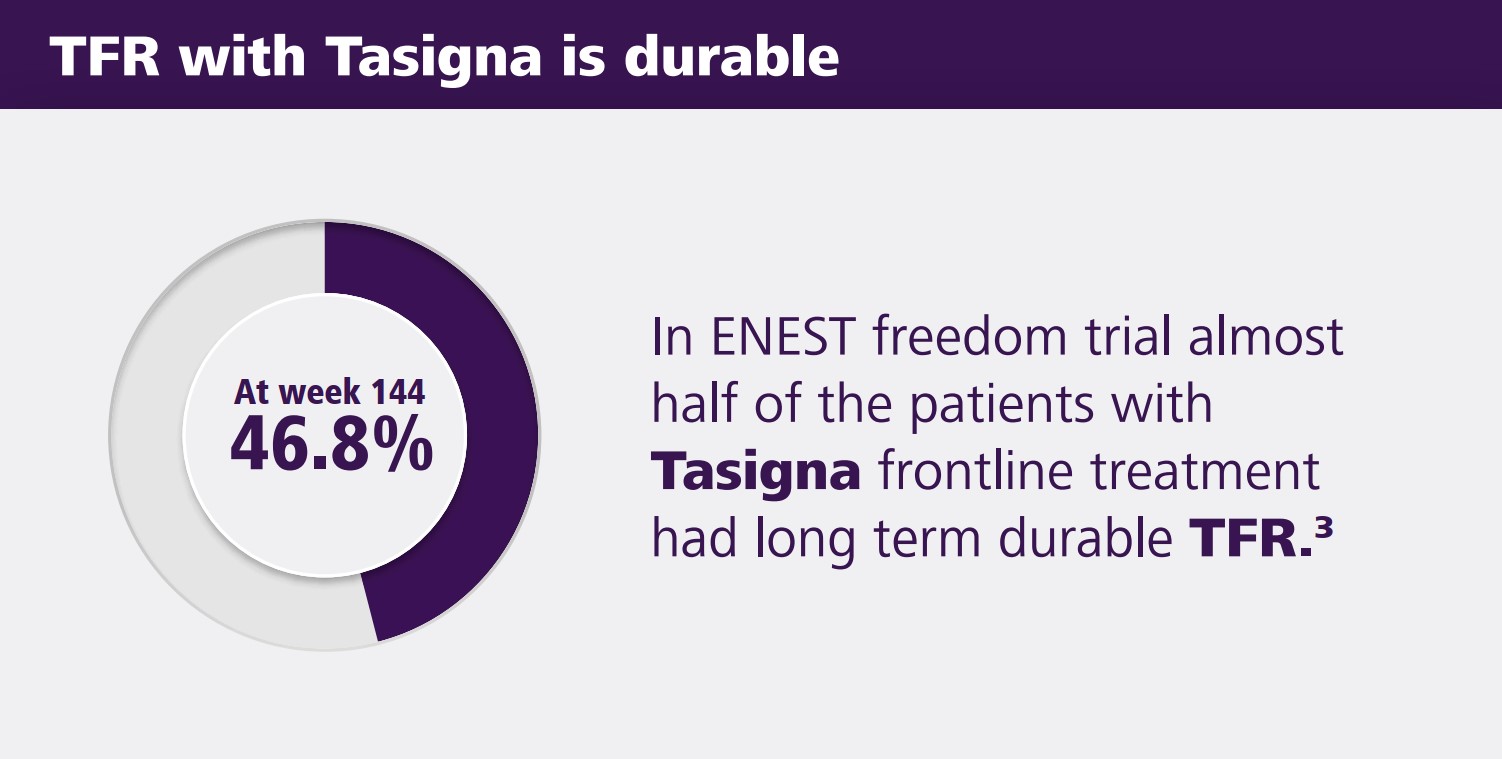
Discontinuation of treatment may be considered in eligible Ph+ CML-CP patients who have been treated with Tasigna for a minimum of 3 years if a deep molecular response (MR 4.5) is sustained for a
minimum of one year prior to discontinuation of therapy. Discontinuation of Tasigna should be initiated by a physician experienced in the treatment of patients with CML. Patients who are eligible to
discontinue Tasigna therapy must have their BCR-ABL transcript levels and complete blood count with differential monitored monthly for one year, then every 6 weeks for the second year and every 12
weeks thereafter.
Monitoring of BCR-ABL transcript levels must be performed with a quantitative diagnostic test validated to measure molecular response levels on the International Scale (IS) with a sensitivity of at least
MR 4.5 (BCR-ABL/ABL ≤0.0032% IS).
For patients who lose MR 4 (MR 4 = BCR-ABL/ABL ≤0.01% IS) but not MMR (MMR = BCR-ABL/ABL ≤0.1% IS) during the treatment-free phase, BCR-ABL transcript levels should be monitored every
2 weeks until BCR-ABL levels return to a range between MR 4 and MR 4.5. Patients who maintain BCR-ABL levels between MMR and MR 4 for a minimum of 4 consecutive measurements can return to
the original monitoring schedule.
Patients who have discontinued Tasigna treatment after achieving a sustained deep molecular response on Tasigna as a first-line TKI treatment and who no longer exhibit a major molecular response
(MMR or MR 3.0) must resume treatment within 4 weeks from the time at which the loss of remission is known to have occurred. Tasigna therapy must be reinitiated at 300 mg twice daily or at a reduced dose level of 400 mg once daily if the patient had their dose reduced before discontinuation of therapy.
Patients who have discontinued Tasigna treatment after achieving a sustained deep molecular response on Tasigna after prior imatinib therapy and in whom there is a confirmed loss of MR 4.0 (two consecutive measurements at least 4 weeks apart showing a loss of MR 4.0) or loss of major molecular response (MMR) must resume treatment within 4 weeks of when the loss of remission is known to have
occurred. Tasigna therapy must be resumed at either 300 mg or 400 mg twice daily.
Patients in whom Tasigna therapy is resumed should have their BCR-ABL transcript values monitored monthly until the previous major molecular response or MR 4.0 is re-established.
Special dosage recommendations
Children and adolescents:
The safety and efficacy of Tasigna in paediatric patients with Ph+ CML-CP aged from 2 to under 18 years have been established. There is no experience in paediatric patients under 2 years of age or in
paediatric patients with Ph+ CML-AP or blast crisis.
Elderly patients:
Approximately 12% and 30%, respectively, of the subjects in clinical studies (newly diagnosed Ph+ CML-CP and resistant or intolerant Ph+ CML-CP and CML-AP) were 65 years of age or older. No
special dose adjustments are necessary in patients over 65 years of age.
Patients with renal impairment:
Clinical studies have not been performed in patients with renal impairment.
As nilotinib and its metabolites are renally excreted only to a minor extent, a decrease in total body clearance is not anticipated in patients with renal impairment. No dose adjustment is necessary in
patients with renal impairment.
Patients with hepatic impairment:
The pharmacokinetic parameters of nilotinib are moderately affected in patients with mild to severe hepatic impairment. Dose adjustment is not considered necessary in patients with hepatic impairment. Caution should nevertheless be exercised when treating these patients. Treatment is not recommended in patients in whom transaminases are elevated by 2.5 times the norm or bilirubin by 1.5
times the norm. Serum lipase levels should be measured monthly or as clinically indicated.
Contraindications: Hypersensitivity to nilotinib or to any of the excipients.
Warnings and precautions: Treatment with TASIGNA associated with thrombocytopenia, neutropenia and anemia. Complete blood counts to be performed every two weeks for the first 2 months and
then monthly thereafter or as clinically indicated. Caution in patients who have or may develop prolongation of QTc (e.g., patients with hypokalemia, hypomagnesemia; with uncontrolled or significant
cardiac disease including recent myocardial infarction, congestive heart failure, unstable angina or clinically significant bradycardia; patients taking anti-arrhythmic medicines or other drugs that may
lead to QT prolongation). Hypokalemia or hypomagnesemia must be corrected prior to TASIGNA administration. Uncommon cases (0.1 to 1%) of sudden death have been reported in clinical trials
in patients with significant cardiac risk factors (including ventricular repolarization abnormalities) or with comorbidities/concomitant medications (not in the newly diagnosed Ph+ CML-CP study). The
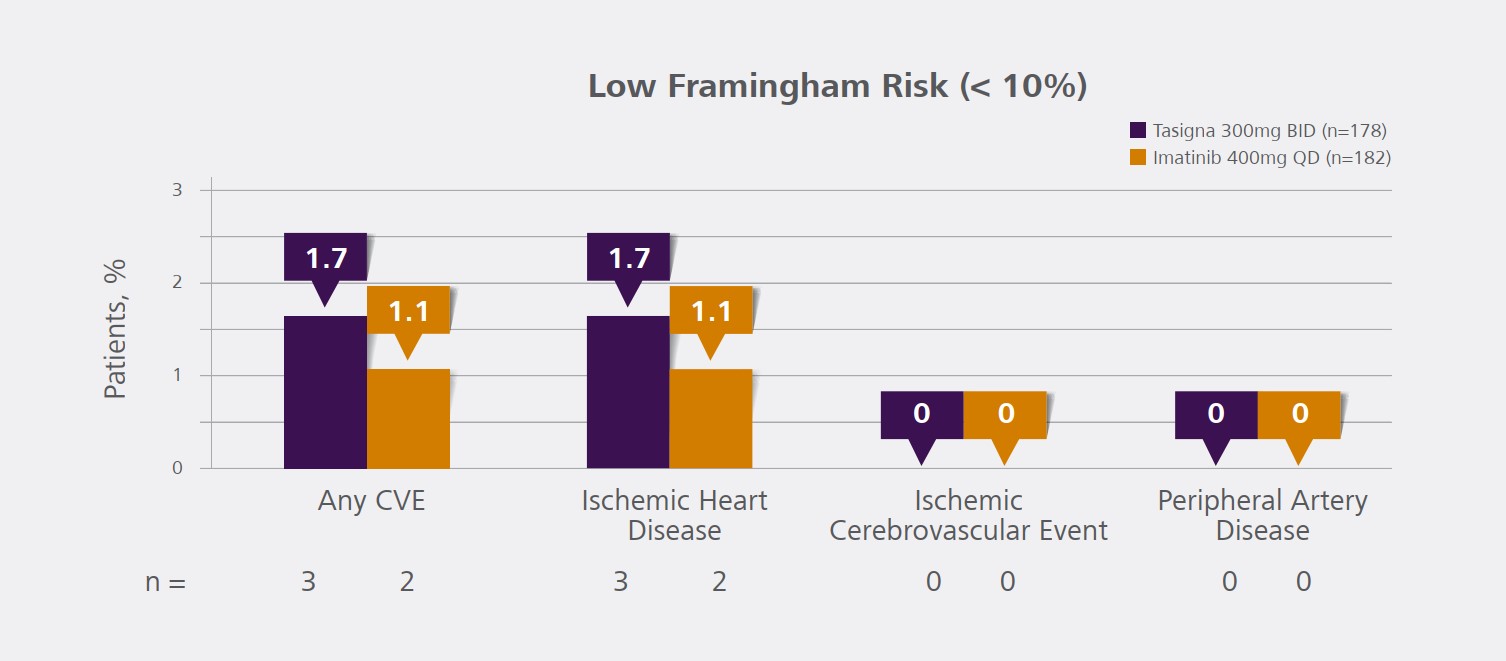
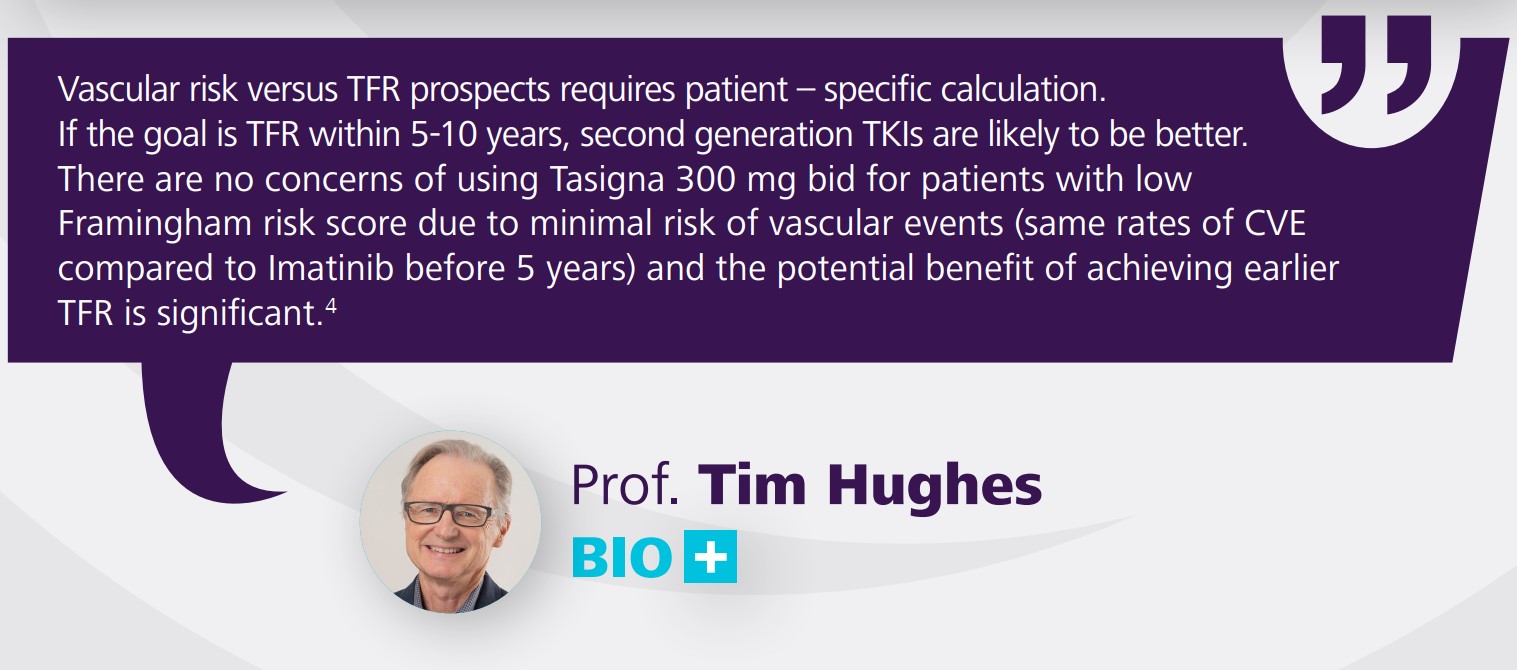
estimated reporting rate for spontaneous reports of sudden death is 0.02% per patient-year. Cardiovascular events (peripheral arterial occlusive disease, ischemic heart disease and ischemic cerebrovascular events) were reported in newly diagnosed CML patients and observed in the post-marketing reports. If acute signs or symptoms of cardiovascular events occur, advise patients to seek immediate
medical attention. The cardiovascular status of patients should be evaluated and cardiovascular risk factors should be monitored and actively managed during TASIGNA therapy according to standard
guidelines. Unexpected, rapid weight gain should be carefully investigated. If signs of severe fluid retention appear during treatment with nilotinib, the etiology should be evaluated and patients treated
accordingly. It is recommended that the lipid profiles be determined before initiating treatment with TASIGNA, assessed at the first few months after initiating therapy. If a HMG-CoA reductase inhibitor (a lipid lowering agent) is needed, refer to Interactions section , before starting treatment since certain HMG-CoA reductase inhibitors are metabolized by the CYP3A4 pathway. Blood glucose levels
should be assessed before initiating treatment with TASIGNA. Test for hepatitis B infection before initiating treatment with TASIGNA. In patients with positive hepatitis B serology (including those
with active disease) and for patients who test positive for hepatitis B infection during treatment, consult experts before initiating treatment. Closely monitor for signs and
symptoms of active hepatitis B infection in carriers of hepatitis B virus throughout therapy and for several months following termination of therapy. Must not be taken with food. In pediatric patients
the long-term effects of prolonged treatment with TASIGNA is unknown. Monitoring of BCR-ABL transcript levels in patients eligible for treatment discontinuation must be performed with a quantitative diagnostic test validated to measure molecular response levels with a sensitivity of at least MR4.5. BCR-ABL transcript levels must be assessed prior to and during treatment discontinuation.
Frequent monitoring of BCR-ABL transcript levels and complete blood count with differential is required to detect possible loss of remission. Avoid grapefruit juice and other foods that are known to
inhibit CYP3A4. Caution in patients with previous history of pancreatitis. Interrupt treatment in case of lipase elevations accompanied by abdominal symptoms. The bioavailability of nilotinib might
be reduced in patients with total gastrectomy. Due to possible occurrence of tumor lysis syndrome, correction of clinically significant dehydration and treatment of high uric acid levels are recommended prior TASIGNA administration.
Pregnancy: Women of child-bearing potential must use an effective method of contraception while receiving TASIGNA and for up to 2 weeks after ending treatment. Should not be used during pregnancy unless clearly necessary.
Breast-feeding: Women taking TASIGNA should not breast-feed while taking TASIGNA and for 2 weeks after the last dose.
Interactions: Avoid in patients treated with medicines known to prolong the QT interval (e.g., chloroquine, methadone, halofantrine, clarithromycin, haloperidol, moxifloxacin, bepridil, pimozide).
Avoid in patients treated with anti-arrhythmic medicines (e.g., amiodarone, disopyramide, procainamide, quinidine, sotalol). Avoid administration of strong CYP3A4 inhibitors (e.g., ketoconazole, ritonavir, itraconazole, voriconazole, telithromycin). Caution with CYP3A4 inducers (e.g., phenytoin, rifampicin, carbamazepine, phenobarbital, or St. John’s Wort). TASIGNA may be used concurrently
with esomeprazole or other proton pump inhibitors. TASIGNA can be used concurrently with warfarin. Caution with medicines that affect P-glycoprotein. Nilotinib is a moderate CYP3A4 inhibitor.
The systemic exposure of other drugs primarily metabolized by CYP3A4 (e.g., certain HMG-CoA reductase inhibitors) may be increased when co-administered with nilotinib. Appropriate monitoring
and dose adjustment may be necessary for drugs that are CYP3A4 substrates and have a narrow therapeutic index (including but not limited to alfentanil, cyclosporine, dihydroergotamine, ergotamine,
fentanyl, sirolimus and tacrolimus) when co-administered with nilotinib. Avoid grapefruit juice and other foods that are known to inhibit CYP3A4. In concurrent use: the H2 blocker (e.g., famotidine)
may be administered approximately 10 hours before and approximately 2 hours after TASIGNA dose; antacids (e.g., aluminum hydroxide, magnesium hydroxide, simethicone) may be administered approximately 2 hours before or approximately 2 hours after TASIGNA dose.
Adverse drug reactions:
Very common (≥10%): headache, nausea, constipation, diarrhoea, vomiting, rash, pruritus, alopecia, dry skin, myalgia, fatigue, hypophosphataemia (including blood phosphorus decreased), hyperbilirubinaemia , alanine aminotransferase increased, aspartate aminotransferase increased, lipase increased, lipoprotein cholesterol (including low density and high density) increased, total cholesterol
increased, blood triglycerides increased, myalgia, bone pain.
Common (1 to 10%): abdominal pain upper, bone pain ,arthralgia,musculoskeletal pain,arthralgia, neutropenia, folliculitis, upper respiratory tract infection (including pharyngitis, nasopharyngitis,
rhinitis), skin papilloma, leukopenia, eosinophilia, febrile neutropenia, pancytopenia, lymphopenia, electrolyte imbalance (including hypomagnesaemia, hyper/hypokalaemia, hyponatraemia, hyper/
hypocalcaemia, hyperphosphataemia), diabetes mellitus, hyperglycaemia, hypercholesterolaemia, hyperlipidaemia, decreased appetite, anorexia, depression, insomnia, anxiety, dizziness, peripheral
neuropathy, hypoaesthesia, paraesthesia, eye haemorrhage, periorbital oedema, eye pruritus, conjunctivitis, dry eye (including xerophthalmia), vertigo, angina pectoris, arrhythmia (including atrioventricular block, cardiac flutter, extrasystoles, atrial fibrillation, tachycardia, bradycardia), palpitations, electrocardiogram QT prolonged, hypertension, flushing, dyspnoea, dyspnoea exertional, epistaxis,
cough, dysphonia, pancreatitis, abdominal discomfort, dyspepsia, dysgeusia, flatulence, hepatic function abnormal, night sweats, eczema, urticaria, hyperhidrosis, contusion, acne, dermatitis (including
allergic exfoliative and acneiform), muscle spasms, bone pain, pain in extremity, musculoskeletal chest pain, musculoskeletal pain, back pain, neck pain, flank pain, muscular weakness, pollakiuria,
oedema peripheral, chest pain (including non-cardiac chest pain), pain, chest discomfort, malaise, haemoglobin decreased, gamma-glutamyltransferase increased, blood creatine phosphokinase increased, blood alkaline phosphatase increased, blood insulin increased, weight increased.
Uncommon (0.1 to 1%): , globulins decreased pneumonia, urinary tract infections, asthenia, blood amylase increased, gastroenteritis, bronchitis, herpes virus infection, candidiasis including oral candidiasis, hyperthyroidism, hypothyroidism, gout, dehydration, increased appetite, dyslipidaemia, intracranial haemorrhage, ischaemic stroke, transient ischaemic attack, cerebral infarction, migraine,
loss of consciousness (including syncope), tremor, disturbance of attention, hyperaesthesia, vision impairment, vision blurred, visual acuity reduced, eyelid oedema, photopsia, hyperaemia (scleral,
conjunctival, ocular), eye irritation, conjunctival haemorrhage, cardiac failure, myocardial infarction, coronary artery disease, cardiac murmur, pleural and pericardial effusions, cyanosis, hypertensive
crisis, peripheral arterial occlusive disease, intermittent claudication, arterial stenosis limb, haematoma, arteriosclerosis, pulmonary oedema, interstial lung disease, pleuric pain, pleurisy, pharyngolaryngeal pain, throat irriation, gastrointestinal haemorrhage, melena, mouth ulceration, gastroesophageal reflux, stomatitis, oesophageal pain, dry mouth, gastritis, sensitivity of teeth, hepatotoxicity,
toxic hepatitis, jaundice, exfoliative rash, drug eruption, pain of skin, ecchymosis, swelling face, musculoskeletal stiffness, joint swelling, dysuria, micturation urgency, nocturia, breast pain, gynaecomastia, erectile dysfunction, face oedema, influenza-like illness, chills, feeling body temperature change (including feeling hot, feeling cold),
Frequency not known: hepatitis B reactivation.
Packs and prices: Country-specific.
Legal classification: Country-specific.
BSS Version: 2.0
Leaflet revision Date: November 2018